Patient Support Line 1-866-433-8000, Monday - Friday: 8:00 AM to 8:00 PM ET

KISQALI + hormone therapy has been proven to help people like you live longer
People with HR+, HER2- metastatic breast cancer (mBC) were treated with KISQALI in multiple studies. In each study, the goal was to determine if KISQALI + hormone therapy could help people live longer than hormone therapy alone.
Leading cancer experts choose ribociclib (KISQALI®)
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), written by leading cancer experts, recommend ribociclib (KISQALI®) + hormone therapy as effective and established treatment for eligible people with HR+, HER2- mBC.
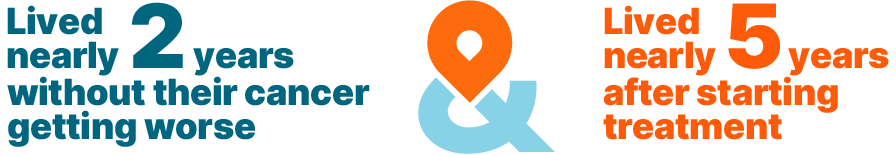
With KISQALI + hormone therapy, half of all patients with HR+, HER2- mBC:
KISQALI is not approved for use with tamoxifen.
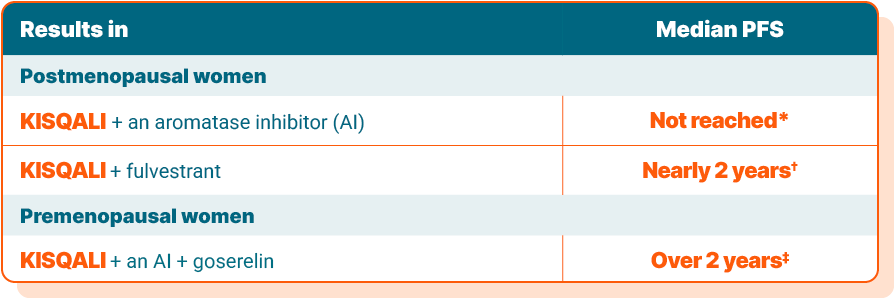
Breaking down the data
In studies, time spent living without cancer getting worse is called progression-free survival, or PFS. The results are presented as median PFS, which is the length of time after starting the study that half of the patients were living without their cancer getting worse.
*In the MONALEESA-2 study of postmenopausal women, median PFS at a 15.3-month check-in was not reached for KISQALI + an AI vs 14.7 months with placebo + an AI. This means that at the time of analysis, more than half of the patients taking KISQALI + an AI were living without their disease spreading or worsening.
†In the MONALEESA-3 study of postmenopausal women, median PFS at a 20-month check-in was 20.5 months with KISQALI + fulvestrant vs 12.8 months with placebo + fulvestrant.
‡In the MONALEESA-7 study of premenopausal women, median PFS at a 19-month check-in was 27.5 months with KISQALI + an AI + goserelin vs 13.8 months with placebo + an AI + goserelin.
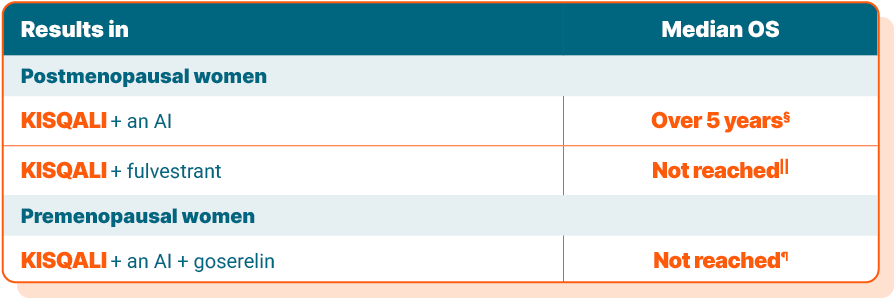
Time spent living with breast cancer is called overall survival, or OS. The results are presented as median OS, which is the length of time after starting the study that half of the patients were still living.
§In the MONALEESA-2 study of postmenopausal women, median OS at an 80-month check-in was 63.9 months with KISQALI + an AI vs 51.4 months with placebo + an AI.
||In the MONALEESA-3 study of postmenopausal women, median OS at a 39-month check-in was not reached for KISQALI + fulvestrant vs 40 months with placebo + fulvestrant. This means that at the time of the analysis, more than half of the patients taking KISQALI + fulvestrant were still alive.
¶In the MONALEESA-7 study of premenopausal women, median OS at a 35-month check-in was not reached for KISQALI + an AI + goserelin vs 40.7 months with placebo + an AI + goserelin. This means that at the time of analysis, more than half of the patients taking KISQALI + an AI + goserelin were still alive.
Results with KISQALI glossary
Learn more about key terms used on this page by tapping or clicking on the words below.